10N.1.SL.TZ0.23
Core, Topic 7: Atomic, nuclear and particle physics, 7.3 – The structure of matter
The Geiger–Marsden experiment provides evidence for
A. the existence of discrete atomic energy levels.
B. the existence of the neutron.
C. a dense positively charged nucleus.
D. the stability of some nuclei.
AA265
Topic 3—Geometry and trigonometry, SL 3.5—Unit circle definitions of sin, cos, tan. Exact trig ratios, ambiguous case of sine rule, SL 3.6—Pythagorean identity, double angles, SL 3.7—Circular functions: graphs, composites, transformations, SL 3.8—Solving trig equations
15M.2.HL.TZ2.9
Core, Topic 4: Chemical bonding and structure, 4.1 Ionic bonding and structure
Consider the structure and bonding in and .
Consider the molecules and .
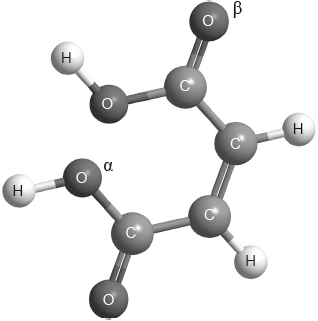
The structure of cis-but-2-ene-1,4-dioic acid is shown below.
a.i. State and explain the electrical conductivities of these two chloride compounds in their liquid state. [3]
a.ii. Suggest, giving your reasons, the approximate pH values of the solutions formed by adding each chloride compound separately to distilled water.
[4]
b.i. Identify the acid-base character of the oxides of each of the elements from sodium to chlorine in period 3. [2]
b.ii. State the equations for the separate reactions of sodium oxide and phosphorus(V) oxide with water. [2]
c.i. Deduce the Lewis (electron dot) structure of both molecules. [2]
c.ii. Predict the shapes of the two molecules, giving the Br–P–Br bond angle in and the F–S–F bond angles in .
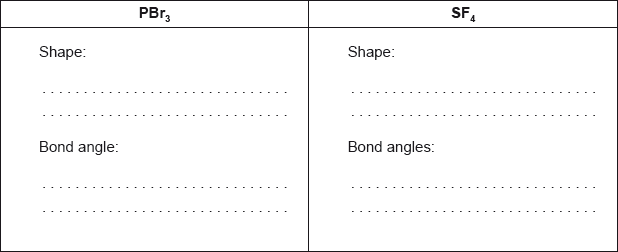 [4]
[4]
c.iii. Explain why both and are polar. [2]
d.i. Describe the covalent bond between carbon and hydrogen in the molecule above and how it is formed. [2]
d.ii. Deduce the hybridization of the oxygen atoms labelled and .
:
: [1]
d.iii. Describe sigma and pi bonds between atoms.
bond:
bond: [2]
d.iv. Identify the number of sigma and pi bonds present in a molecule of cis-but-2-ene-1,4-dioic acid. [1]